
New Drug Designations - May 2024
Shots:
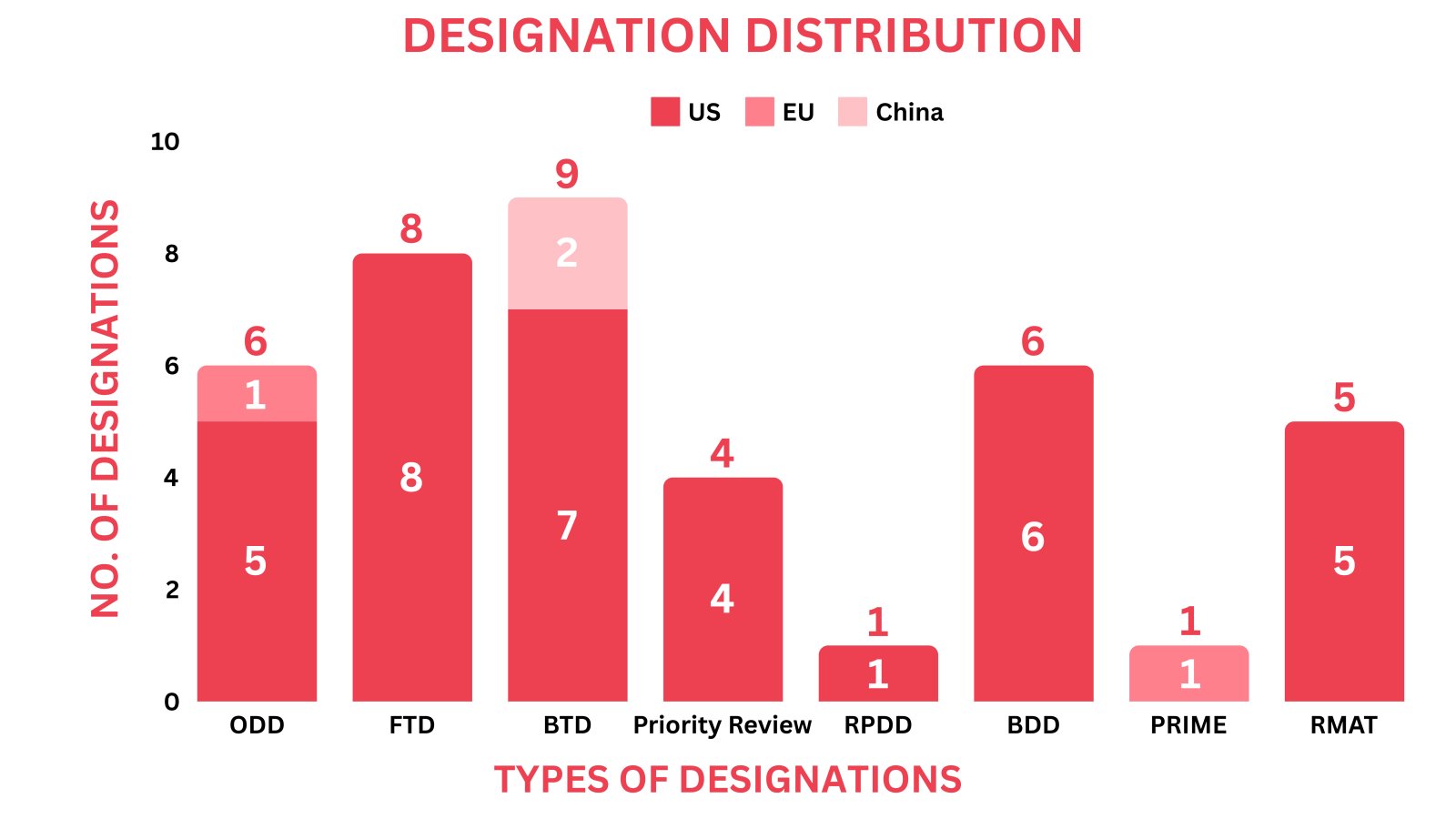
- PharmaShots' designation report provides a concise overview of several drugs and their designations by the FDA, NMPA and EMA. This month’s report includes designations allotted to 12 small molecules, 7 biologics, 9 cell & gene therapies, 1 vaccine, 1 peptide, 1 RNA candidate, 1 recombinant fusion protein, 1 imaging agent and 6 devices
- This month, Roche’s Inavolisib received US FDA’s BTD & Priority Review for Breast Cancer, Wugen’s WU-CART-007 gained FDA’s RMAT & EMA’s PRIME designation for T-Cell Acute Lymphoblastic Leukemia/Lymphoma as well as SN Bioscience’s SNB-101 was designated with EMA’s ODD & FDA’s FTD for SCLC
- PharmaShots has compiled a list of a total of 33 drugs and 6 devices awarded with designations by multiple regulatory bodies in May 2024

Azeliragon
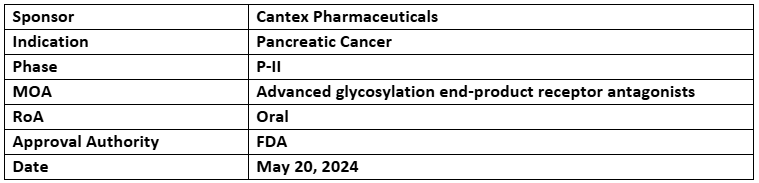
- The company is currently recruiting patients for P-II trial evaluating the safety and efficacy of azeliragon to treat metastatic pancreatic cancer who are refractory to 1L therapy across the US
- Azeliragon has already received ODD from FDA for Glioblastoma in 2023
- Cantex gained global rights of Azeliragon from vTv therapeutics in Jun 2021 for oncology whereas vTv is assessing it Alzheimers and other indications beyond oncology
SPG601

- The FDA granted ODD to SPG601 for the treatment of Fragile X Syndrome
- SPG601 is a BK channel activator that binds to and increases activated BK channels to restore synaptic function.
SurVaxM
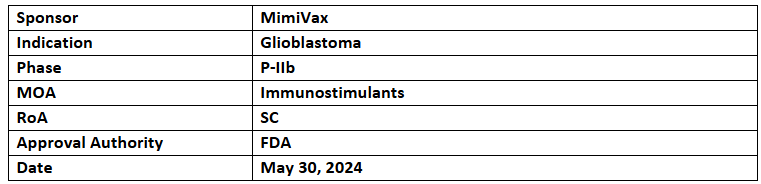
-
The P-IIb (SURVIVE) trial evaluates the efficacy of SurVaxM for treating newly diagnosed glioblastoma (nGBM)
Vorinostat (RVL-001)
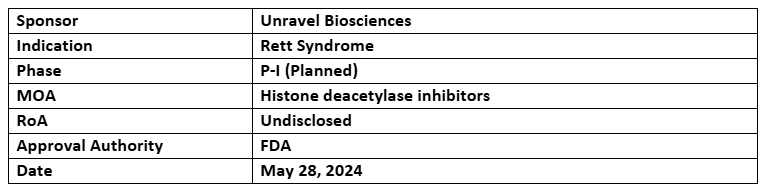
- The PoC study of RVL-001, developed using the BioNAV platform, received positive pre-IND feedback from the US FDA. Its manufacturing for future studies across the US and Colombia has begun in Mar 2024
- Unravel partnered with Vanderbilt University to conduct the US clinical study of RVL-001 for treating Rett syndrome
CAN-3110
- CAN-3110 is being assessed under the P-Ib study for recurrent high-grade glioma (rHGG)
- The results of arm A, published in Nature, depicted robust anti-tumoral activity resulting in extended survival. Feasibility and safety data of multiple doses, supported by the Break Through Cancer Foundation, was highlighted at ASCO 2024
- CAN-3110 is a replication-competent herpes simplex virus-1 (HSV-1) oncolytic viral immunotherapy possessing oncolysis and immune activation in one treatment
SNB-101
- The EMA granted ODD to SNB-101 for small cell lung cancer (SCLC) and received 10-year exclusive rights. It was designated with the US FDA’s ODD for SCLC in Jul 2023 and FTD in May 2024 & pancreatic cancer in Feb 2024
- The P-II study of SNB-101 has been concluded & the IND for P-II was approved in Korea in Sep 2023. P-II study planned post-IND approval in the US in July 2024 July 2014
- SNB-101, nanoparticle anticancer drug containing SN-38, showed favorable efficacy & reduction over existing treatment

Eneboparatide
- The US FDA has granted fast track designation to the company’s eneboparatide for treating hypoparathyroidism
- Eneboparatide is being developed under the P-III (Calypso) trial to investigate its safety & efficacy for treating chronic hypoparathyroidism patients (n=165) across 50 sites in US, EU, Canada, UK. The topline results are anticipated in 2025
- Eneboparatide works by binding with the PTH receptor to stabilize blood calcium levels and reduce urine calcium excretion thus managing the hypoparathyroidism symptoms. It aims at preventing kidney function decline and chronic kidney disease development
APN-1607
- The FTD granted to APN-1607 [florzolotau (18F)] is intended for detecting tau protein among patients with suspected progressive supranuclear palsy (PSP)
- The recruitment for P-III trial of APN-1607 for Alzheimer's diagnosis was completed across China with the US FDA’s “Study May Proceed” letter to conduct its global P-III trial to diagnose PSP
- APN-1607 is a radioactive PET imaging molecule to visualize & measure tau aggregates (3R and 4R) in disorders like PSP, frontotemporal dementia & Alzheimer's disease
SNB-101
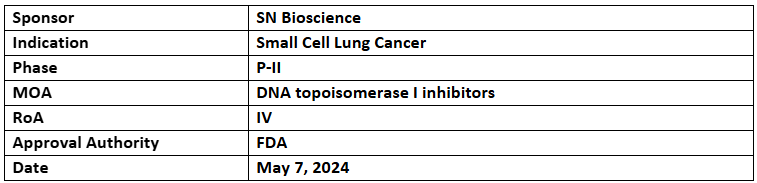
- The US FDA has granted FTD to SNB-101 for small cell lung cancer (SCLC). It was designated with the US FDA’s ODD for SCLC in Jul 2023 & pancreatic cancer in Feb 2024
- The P-I study of SNB-101 has been concluded & the IND for P-II is approved in Korea with global P-II study planned post IND approval across the US & EU in H2’24. The company also anticipates indication expansion to other solid tumors incl. colon cancer, gastric cancer & biliary tract cancer that will be confirmed via P-II study
- SNB-101, nanoparticle anticancer drug containing SN-38, showed favorable efficacy & reduction in digestive system AEs vs existing anticancer drugs in preclinical & P-I evaluations
ECUR-506
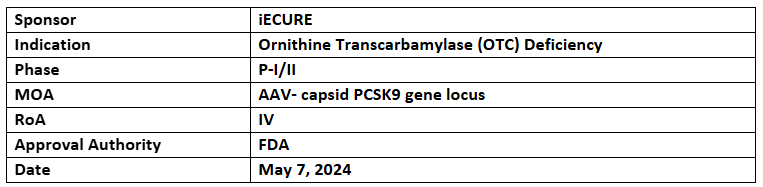
- The US FDA granted FTD for the treatment of neonatal onset Ornithine Transcarbamylase (OTC) deficiency
- ECUR-506 is being investigated under P-I/II (OTC-HOPE) study for its safety, efficacy, PK and tolerability among newborn males with genetically confirmed neonatal onset OTC deficiency. Recruitment is ongoing in UK with future enrollments across the US & Australia
TPN-101

- The US FDA has granted fast-track designation to the company’s TPN-101 for Progressive Supranuclear Palsy
- The FTD was based on the P-II study of TPN-101 for 48 wks. The study shows stable clinical improvement for symptoms as measured by the PSPRS between 24 and 48 wks.
- TPN-101 selectively inhibits LINE-1 reverse transcriptase, which enhances LINE-1 replication and lower the levels of NfL and IL-6 Levels, Important Biomarkers of Neurodegeneration and Neuroinflammation in PSP
VCN-01
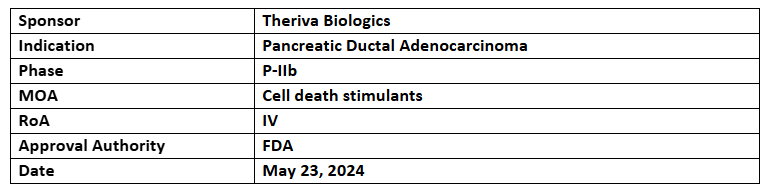
- The US FDA has granted fast-track designation to the company’s VCN-01 (IV) + CT (gemcitabine/nab-paclitaxel) for PDAC and previously received ODD
- The company’s VCN-01 is being evaluated in P-IIb (VIRAGE) study to treat metastatic PDAC; patient enrolment completion is anticipated in Q3’24
- VCN-01, an oncolytic adenovirus administered to over 80 patients in P-I and investigator-sponsored trials to treat PDAC (in combination with chemotherapy), HNSCC (with an immune checkpoint inhibitor), ovarian cancer (with CAR-T cell therapy), colorectal cancer, and retinoblastoma (by intravitreal injection)
AFM24
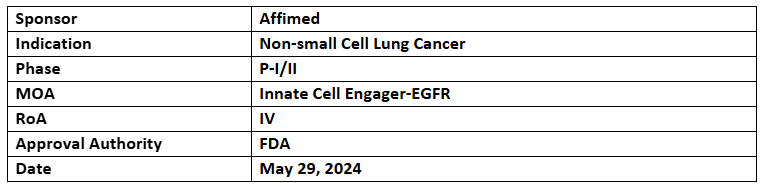
- The US FDA has granted FTD to AFM24 in combination with atezolizumab to treat advanced and/or metastatic, EGFR wild-type, non-small cell lung cancer (NSCLC) patients whose disease progressed post PD-(L)1 therapy and Pt-based CT
- The FTD was based on the P-II part of P-I/II (AFM24-102) study of AFM24 in addition to atezolizumab for its efficacy in certain advanced/metastatic EGFR-expressing cancers
RAG-01

- The company is currently assessing RAG-01 in the P-I study to treat NMIBC in Australia. It has recruited and dosed 3 patients after initiating recruitment in Dec’23
- US FDA along with designation also approved IND application to initiate P-I in the States
- RAG-01 is an saRNA candidate that activates the tumor suppressor gene p21 through the RNAa mechanism

Delpacibart Etedesiran (AOC 1001)
- It has been previously designated with the US FDA’s ODD & FTD as well as EMA’s ODD for treating DM1
- The company is planning to conduct P-III (HARBOR) trial of del-desiran in Q2’24 having 1EP of video hand opening time (vHOT) and 2EPs are muscle strength as measured by hand grip strength & QMT total score as well as activities of daily living as measured by DM1-Activ
- Its previous long-term MARINA-OLE study showed reversal of disease progression among DM1 adults in endpoints incl. vHOT, muscle strength & DM1-Activ vs natural history data
Scemblix
- The US FDA has granted BTD to Scemblix (asciminib) as a treatment of newly diagnosed Philadelphia chromosome +ve CML in chronic phase (Ph+ CML-CP)
- The designation was based on P-III (ASC4FIRST) trial that reached its 1EPs and showed superior MMR rates at wk.48 vs SoC TKIs (imatinib, nilotinib, dasatinib & bosutinib) vs. imatinib alone with favorable safety profile. Data was highlighted at ASCO 2024
Inavolisib

- The US FDA has granted BTD to inavolisib + Ibrance & fulvestrant based on the P-III (INAVO120) study assessing its safety & efficacy vs PBO for treating PIK3CA-mutated, HR+, HER2-ve, locally advanced/metastatic breast cancer patients (n=325). Results have also been submitted to the EMA and other global health authorities
- The data demonstrated 57% PFS with inavolisib-based regimen vs palbociclib & fulvestrant alone (15.0mos. vs 7.3mos.) and an immature OS, although it favoured a positive trend. Follow-up for OS continues
- The company is currently assessing inavolisib in several combinations under a series of P-III (INAVO120, INAVO121, INAVO122) trials for treating PIK3CA-mutated locally advanced or metastatic breast cancer
Atacicept
- The designation was supported by P-IIb (ORIGIN) study depicting eGFR stabilization at 72wks.
- The P-IIb/III (ORIGIN 3) study assesses the safety & efficacy of atacicept vs PBO with P-IIb being the dose ranging part evaluating 3 doses (150mg, 75mg, 25mg, QW) for 36wks. followed by 150mg for another 60wks.
- The P-III part investigates the safety and efficacy of atacicept (150mg, QW) in IgAN patients (n=188) with persistent proteinuria, despite being on a stable dose of RAS inhibitors (ACEi or ARB) for at least 12wks.
Petosemtamab
- The BTD was granted to petosemtamab for treating recurrent/metastatic HNSCC progressed post Pt-based CT & anti-PD-1/PD-L1 treatment. It received FTD for the same in Aug 2023. A P-III study in previously treated HNSCC and a P-III trial of petosemtamab + Keytruda in treatment-naïve patients is planned in mid-2024
- The designation was based on the ongoing P-I/II study assessing the efficacy, durability & safety of petosemtamab alone to treat advanced solid tumors incl. recurrent/metastatic HNSCC. Data is anticipated in H2’24
- Petosemtamab is a Biclonics low-fucose IgG1 Ab that targets EGFR & LGR5 and shows EGFR-dependent signal inhibition, LGR5 binding leading to EGFR internalization for cancer cell degradation as well as ADCC & ADCP activities
NVL-655
- The BTD has been granted for locally advanced or metastatic ALK+ NSCLC patients treated with ≥2 ALK tyrosine kinase inhibitors (TKIs)
- BTD was supported by preliminary safety and activity observed in the P-I part of P-I/II (ALKOVE-1) study. Recruitment for P-II part is underway with the data anticipated at a future conference in H2’24
- NVL-655 is a novel brain-penetrant ALK-selective TKI and remains active in tumor with resistance for 1st, 2nd & 3rd generation ALK inh.
Larsucosterol
- BTD has been granted for treating severe alcohol-associated hepatitis (AH)
- The BTD was based on the P-IIb (AHFIRM) study assessing the safety & efficacy of larsucosterol (30mg, 90mg) vs PBO for treating severe AH patients (n=307). This is a global study with recruitment ongoing US, EU, UK & Australia
- Topline data was reported in 2023 and updated results were highlighted at EASL 2024
BRII-877 and BRII-835
- The company’s BRII-877 (tobevibart) and BRII-877 (tobevibart), targeting HBV, have received BTD by the NMPA
- BRII-877's BTD was based on P-I & II trials, carried out with Vir Biotechnology, in 350 patients with HBV. Data, as of Sep 2023, depicted reduced HBsAg levels, indicating its potential as a treatment for chronic HBV and HDV infections
- BRII-835's BTD was supported by P-I & II studies, carried out with Vir Biotechnology, in >570 patients with HBV that showed the drug’s tolerability and anti-viral activity against HBV & HDV infection
IBI343
- The NMPA has granted BTD to IBI343 as monotx. for the treatment of advanced G/GEJ adenocarcinoma who are claudin18.2-positive and have progressed after at least 2L of prior systematic treatment
- The BTD was based on ongoing P-I trial, evaluating IBI343 monotx. in GC and continue to check efficacy & safety in the registration MRCT. Study results are expected to be published at a upcoming medical conference in 2024
- Innovent, soon to initiate registrational P-III (G-HOPE-001) MRCT of IBI343 to treat GC patients who are claudin18.2-positive, HER2-negative

Upstaza
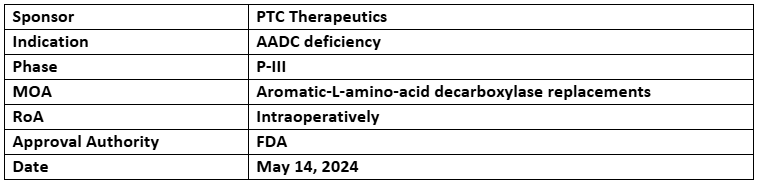
- The US FDA has accepted priority review to the BLA of Upstaza (eladocagene exuparvovec) for treating AADC deficiency.
- On Nov 13, 2024, application has been granted a Priority Review with a target regulatory action
- Upstaza is a one-time recombinant AAV2 based gene therapy containing human DDC gene approved for the treatment of aromatic L–amino acid decarboxylase (AADC) (Age: 18+yrs.)
Inavolisib

- The acceptance & priority review (PDUFA: Nov 27, 2024) for the NDA of inavolisib + Ibrance & fulvestrant was based on P-III (INAVO120, n=325) trial of combination's safety & efficacy vs PBO in PIK3CA-m, HR+, HER2-, locally advanced/metastatic breast cancer post recurrence on/within 12mos. of adjuvant endocrine therapy
- The results depicted more than doubled PFS of 15mos. vs 7.3mos. with a 57% reduced risk of disease worsening/death, OS data was immature, favoring a positive trend & its full assessment is underway. These results will support future global regulatory submissions. Further analysis will be highlighted at ASCO'24
- Inavolisib is under a series of P-III (INAVO120, INAVO121, INAVO122) trials for PIK3CA-m locally advanced or metastatic breast cancer in several combinations
Zanidatamab
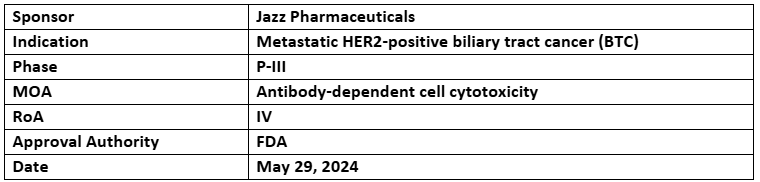
- The US FDA has accepted and granted priority review to the BLA of Zanidatamab for treating metastatic HER2-positive biliary tract cancer (BTC) with a PDUFA date of Nov 29, 2024
- The BLA was supported by the Cohort-1 of P-IIb (HERIZON-BTC-01) trial assessing the safety & efficacy of zanidatamab to treat unresectable, locally advanced, or metastatic HER2+ BTC, results were presented in ASCO 2023 and published in Lancet Oncology, showed 41.3% cORR
- A global confirmatory P-III (HERIZON-BTC-302) study evaluating the efficacy & safety of zanidatamab +SoC vs SoC as a 1L for advanced or metastatic HER2-positive BTC is recruiting
Zenocutuzumab
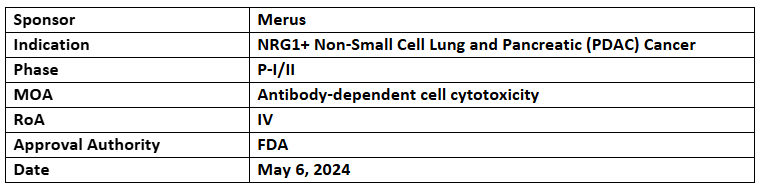
- The US FDA has accepted and granted priority review to the BLA of zenocutuzumab for treating NRG1+ NSCLC and NRG1+ pancreatic (PDAC) cancer
- The BLA was based on clinical data incl. from the P-I/II (eNRGy) study that assessed the safety and anti-tumor activity of zenocutuzumab alone for treating NRG1+ NSCLC, PDAC and other solid tumors
- It has been previously designated with BTD for advanced unresectable or metastatic NRG1+ pancreatic cancer and NSCLC following progression with prior systemic therapy

IB-003
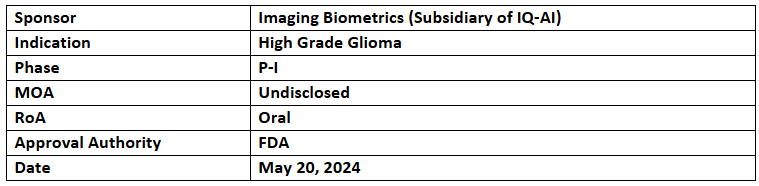
- IB-003 (oral gallium maltolate) has previously received the US FDA’s RPDD for atypical teratoid rhabdoid tumor (ATRT)
- The RPDD designation was based on pre-clinical study that demonstrated growth inhibition and improved survival. mOS was 49 days in the treated group & 21 days in the control group

eLLi Surgical Device
- The US FDA granted BDD to the company’s eLLi surgical device for pediatric patients having Early Onset Scoliosis (EOS) associated with thoracic insufficiency
- eLLi is a non-invasive method to extend growing rods by using increased force, improved mechanical reliability and superior distraction precision
Teal Wand
- The BTD, granted to the device is intended for screening cervical cancer, was based on midpoint study data. It will receive priority review on final data submission
- The test was first assessed in a study involving 215 participants. The company then conducted a global study (SELF-CERV) in >600 diverse participants to confirm its efficacy by comparing the results with clinician-collected samples using a speculum & brush
- The Teal Wand is a device that allows people to collect vaginal sample themselves for cervical cancer screening at home or in a clinic, without an invasive exam
AltaValve System
- The US FDA granted BDD to the company’s transcatheter mitral valve replacement (TMVR) device, AltaValve System, for treating moderate-to-severe or severe mitral regurgitation (MR) and MR with moderate/severe mitral annular calcification (MAC)
- The AltaValve showed high procedural success and complete MR elimination in most patients at 30 days in the Early Feasibility study (NCT03997305).
- 4C Medical plans a global pivotal study in H2’24 for CE mark and FDA approval
Karius Test
- The US FDA has granted BDD to Karius Test for the diagnosis of immunocompromised patients with possible lung infections such as lower respiratory infection & pneumonia
- The test was being compared with SoC under the PICKUP study among adults (n=257) having hematologic malignancies or who underwent hematopoietic cell transplantation with suspected pneumonia
- The study demonstrated that the Karius Test increased the pneumonia causes identification by 40%. It also detected non-pneumonia infections in 39% of individuals, supporting the ATS Workshop Report's observation that current pneumonia definitions lack specificity and highlighting the test's value in identifying infections regardless of pathogen or site
Tina-Quant Lipoprotein Lp(a) RxDx Assay
- The US FDA has granted breakthrough device designation to the company’s Tina-quant lipoprotein Lp(a) RxDx assay for finding out patients who may take advantage from Lp(a)-lowering therapy under development and for HCPs to make precise decisions
- Developed in partnership with Amgen, the test is designed to be utilized in patients with increased lipoprotein Lp(a) and a history of atherosclerotic disease intended to be treated with an Lp(a) lowering drug
- The test works by measuring number of Lp(a) molecules per litre from the patient’s blood sample to serve as an actionable biomarker in the future. It will be available on cobas platforms once approved
Canturio Lumbar Cartridge
- The US FDA has granted BTD to the company’s Canturio Lumbar Cartridge (Canturio LC) with the Canary Health Implanted Reporting Processor (CHIRP) System. The system is designed to be employed with a Lumbar Interbody System for lumbar spinal fusion from L1-S1
- The system gathers and combines data from different parameters assessed for at least 10yrs. providing clinicians with results on activity levels and kinematics, allowing performance comparison by age, gender, and surgery time

WU-CART-007
- The drug has also received the US FDA’s RMAT designation for treating r/r T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL)
- The P-I/II study investigates the safety & efficacy of WU-CART-007 to treat r/r T-ALL/LBL. The data was highlighted at EHA 2024 under posters titled as:
- ‘WU-CART-007, an Allogeneic CAR-T Cell Targeting CD7, is Highly Effective Against Relapsed/Refractory (R/R) T-Cell Acute Lymphoblastic Leukemia/Lymphoma (T-ALL/LBL)
- ‘Preliminary Effect of Enhanced Lymphodepletion (ELD) on WU-CART-007 in T-ALL/LBL’
- ‘WU-NK-101 is an Off-The-Shelf memory NK Cell with a Time-Resilient, Anti-Tumor Phenotype that can be Detected in R/R AML Patient PBMC After Infusion’

TSHA-102
- The US FDA granted RMAT designation to TSHA-102 based on safety & efficacy results from the first 3 Rett syndrome patients administered with its low dose (5.7x10^14 total vg) under REVEAL P-I/II adolescent/adult and pediatric trial
- TSHA-102 being evaluated in P-I/II (REVEAL Adult) dose escalation & expansion trial assessing the safety & preliminary efficacy in adult females with Rett syndrome in Canada & US and another P-I/II (REVEAL Pediatric) trial in pediatric females with Rett syndrome in US & cleared in UK
- TSHA-102 is a self-complementary intrathecal AAV9 gene therapy that delivers functional MECP2 to CNS cells, targeting the genetic root cause of the disease
Descartes-08

- The US FDA has granted RMAT designation to descartes-08 for treating myasthenia gravis (MG). It has also been designated with the FDA’s ODD for the same indication
- The company reported 12mos. follow-up data in Jan 2024 from its P-IIa study. Descartes-08 showed durable autoantibody depletion and improved MG severity scores with a well-tolerated safety profile & without DLTs, cytokine release syndrome or neurotoxicity at 1mos. follow-up period. Topline P-IIb data is anticipated in mid-2024
- Descartes-08, an autologous mRNA CAR-T, works by targeting BCMA and is being developed for MG which is a chronic autoimmune disorder causing muscle weakness and fatigue
TSC-100 and TSC-101
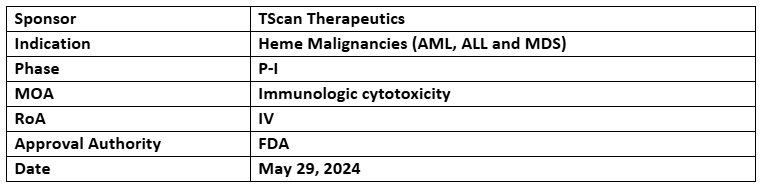
- The US FDA has granted RMAT to two candidates, TSC-100 targeting HA-1 and TSC-101 targeting HA-2, for treating heme malignancies such as acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and myelodysplastic syndrome (MDS)
- The products are being assessed under the P-I study for their feasibility, safety and preliminary efficacy for AML, MDS, or ALL following HCT from a haploidentical donor
- In the P-I study at >10mos. median follow-up, presented in May 2024, patients (n=8) were relapse & disease free without any DLTs; 2/8 relapsed at ~6mos. after transplanting in control arm with 1 death at ~3mos.; another patient in 3rd control arm needed clinical intervention due to impending relapse with 1 death in 4th control arm after transplanting
Bemdaneprocel
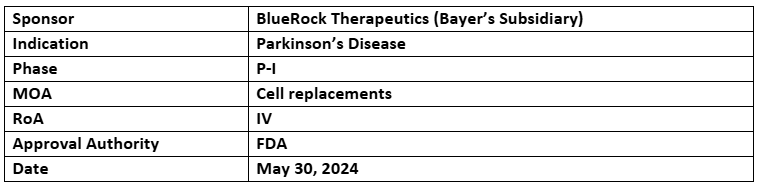
- The P-I study data showed a well-tolerated treatment without any major safety concerns after 18mos & an increased F-DOPA PET signals post stopping immune suppression at 12mos.
- The P-I trial involves 12 Parkinson's patients receiving surgical transplantation of bemdaneprocel cells (Cohort A: 0.9 million cells per putamen, n=5; Cohort B: 2.7 million cells per putamen, n=7) & 1-year immunosuppression regimen
References
- Cantex Pharmaceuticals
- Unravel Biosciences
- Candel Therapeutics
- MimiVax
- SN Bioscience
- Spinogenix
- Ractigen Therapeutics
- Affimed
- Amolyt Pharma
- Theriva Biologics
- iECURE
- APRINOIA Therapeutics
- Transposon Therapeutics
- Vera Therapeutics
- Innovent
- Novartis
- DURECT Corporation
- Brii Biosciences
- Roche 1
- Merus 1
- Avidity Biosciences
- Nuvalent
- Merus 2
- Jazz Pharmaceuticals
- PTC Therapeutics
- Imaging Biometrics
- Teal Health
- Roche 2
- 4C Medical Technologies
- OrthoPediatrics
- Canary Medical
- Karius
- Wugen
- BlueRock Therapeutics
- Taysha Gene Therapies
- Cartesian Therapeutics
- TScan Therapeutics
Related Post: New Drug Designations - April 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














